RAPID REDUCTION OF INFLAMMATION AT DAYS 8 AND 15 VS VEHICLE1
In the same study, significant inflammation reduction was reported as early as Day 3

INFLAMMATION CLEARANCE AT DAY 151,2,4
Additional prespecified endpoint
Zero to trace cells†
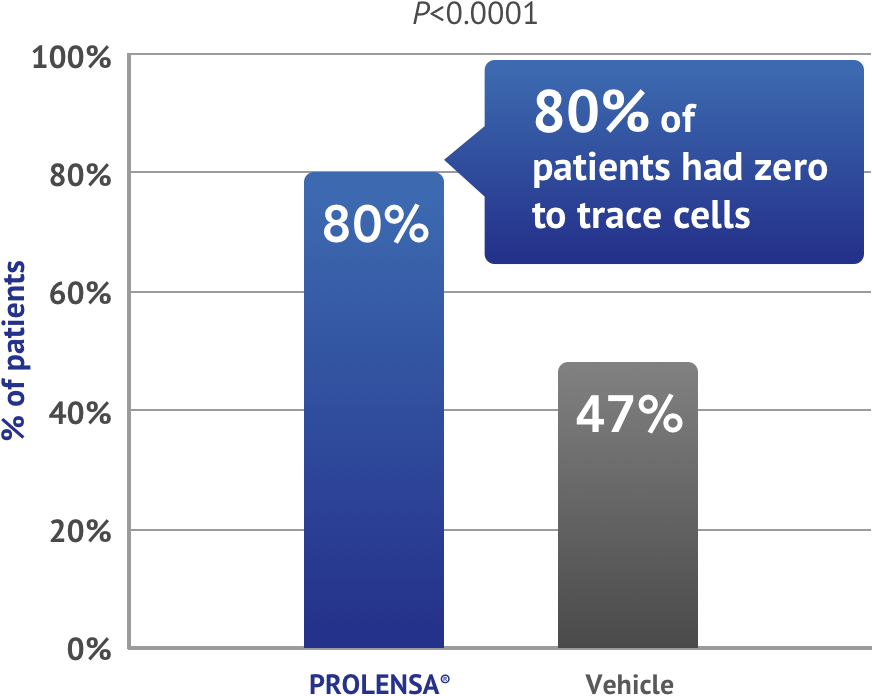
Primary endpoint
Complete clearance of cells and flare*

Study design: Clinical efficacy evaluated in 2 randomized, double-masked, vehicle-controlled trials of patients undergoing cataract surgery. Each randomized patient received PROLENSA® or vehicle starting with one drop into the surgical eye the day prior to and the day of surgery, and for 14 days post-surgery. Study endpoints were clearing of ocular inflammation (SOIS=0) by Day 15 (primary) and the number of subjects pain free on Day 1 after surgery (secondary).1,2
*Ocular inflammation was assessed by the Summed Ocular Inflammation Score (SOIS). Complete clearance of inflammation was defined as the proportion of patients who achieved an SOIS of grade 0 (0 cells and absence of flare).5
†0-5 cells.
PAIN FREE AT DAY 12,4

- 78.8% vs 49.5% with vehicle; P<0.0001
- One dose given on the day of surgery‖
- Only 3% of patients treated with PROLENSA® discontinued by Day 15 due to lack of efficacy vs 24% with vehicle2
‖Dosing: One drop per day starting 1 day prior to surgery, continued on the day of surgery, and through the first 14 days post surgery.2
¶Ocular pain was evaluated by the Ocular Comfort Grading Assessment.2




